Journal of Environmental Chemical Engineering https://doi.org/10.1016/j.jece.2025.119614
Abstract
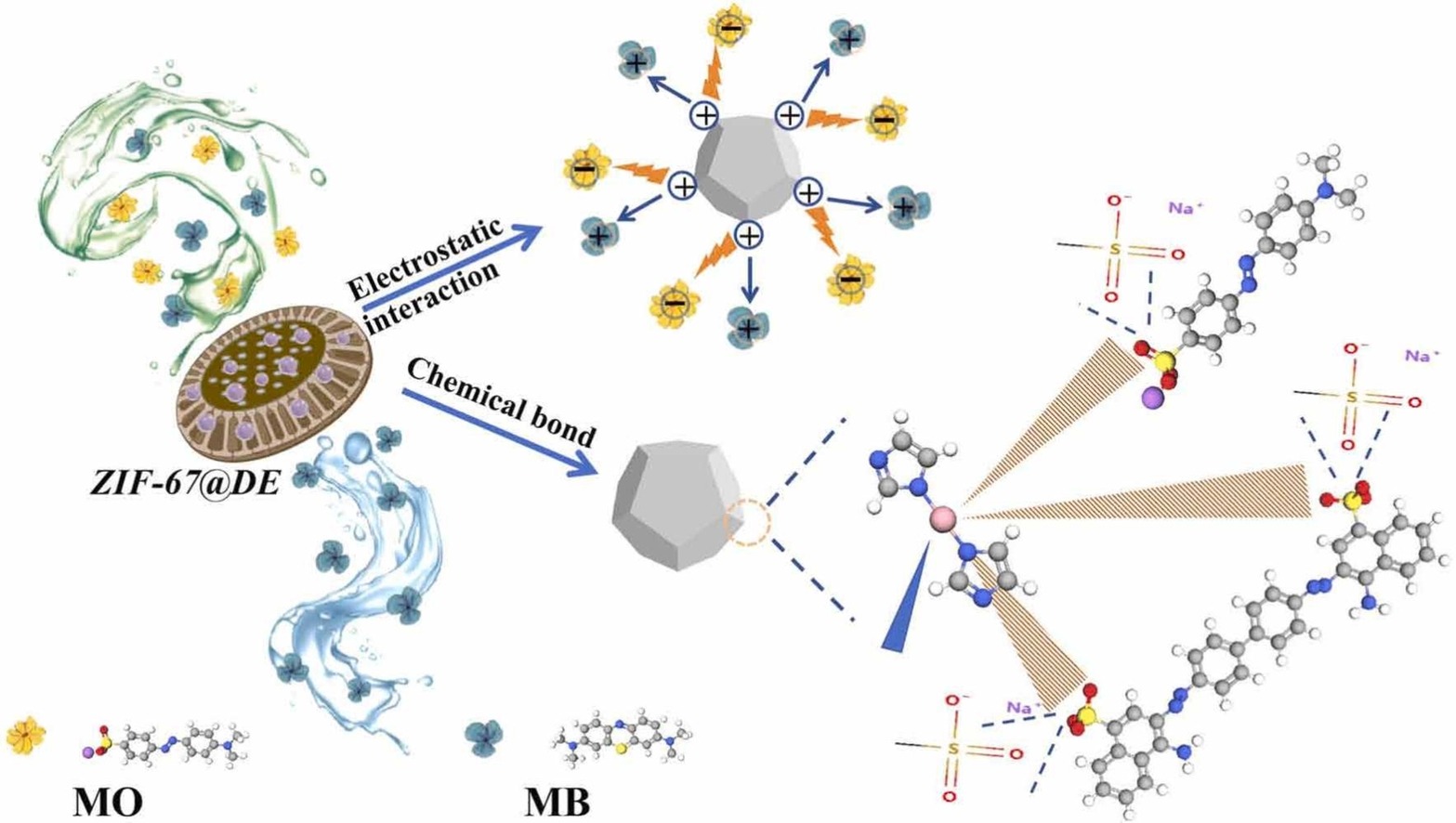
The efficient adsorption and separation of high-concentration mixed dye wastewater has always been a major challenge. In light of this, Zeolite imidazolate framework material (ZIF-67) was rapidly grown in situ on the surface of green, stable and low-cost diatomite (DE) substrate by stirring at room temperature using water as solvent. Interestingly, compared with ZIF-67, the adsorption capacity of diatomite-based composite adsorbent ZIF-67@DE for Congo red (CR) and methyl orange (MO) was significantly improved, and the synthesis yield was increased by 3.73 times. The adsorption process of CR and MO on ZIF-67@DE conformed to the pseudo-second-order kinetics and Langmuir adsorption model, and the maximum adsorption capacities were 1412.6 mg/g and 958.7 mg/g, respectively. ZIF-67@DE was used for the adsorption removal of 11 different types of organic dyes (anions, cations and neutral ions), and it showed consistent high adsorption performance in the adsorption process of sulfonic acid group anionic dyes. Density functional theory (DFT) calculations revealed substantially more negative adsorption energies for anionic dyes compared to cationic dyes on ZIF-67@DE, indicating stronger interactions and rationalizing the observed selectivity. Characterization of the adsorbent before and after dye uptake elucidated the underlying adsorption mechanisms. These results highlight the potential of ZIF-67@DE for the selective separation and preferential adsorption of specific dyes from complex wastewater streams.