Journal of Environmental Chemical Engineering https://doi.org/10.1016/j.jece.2025.118314
Abstract
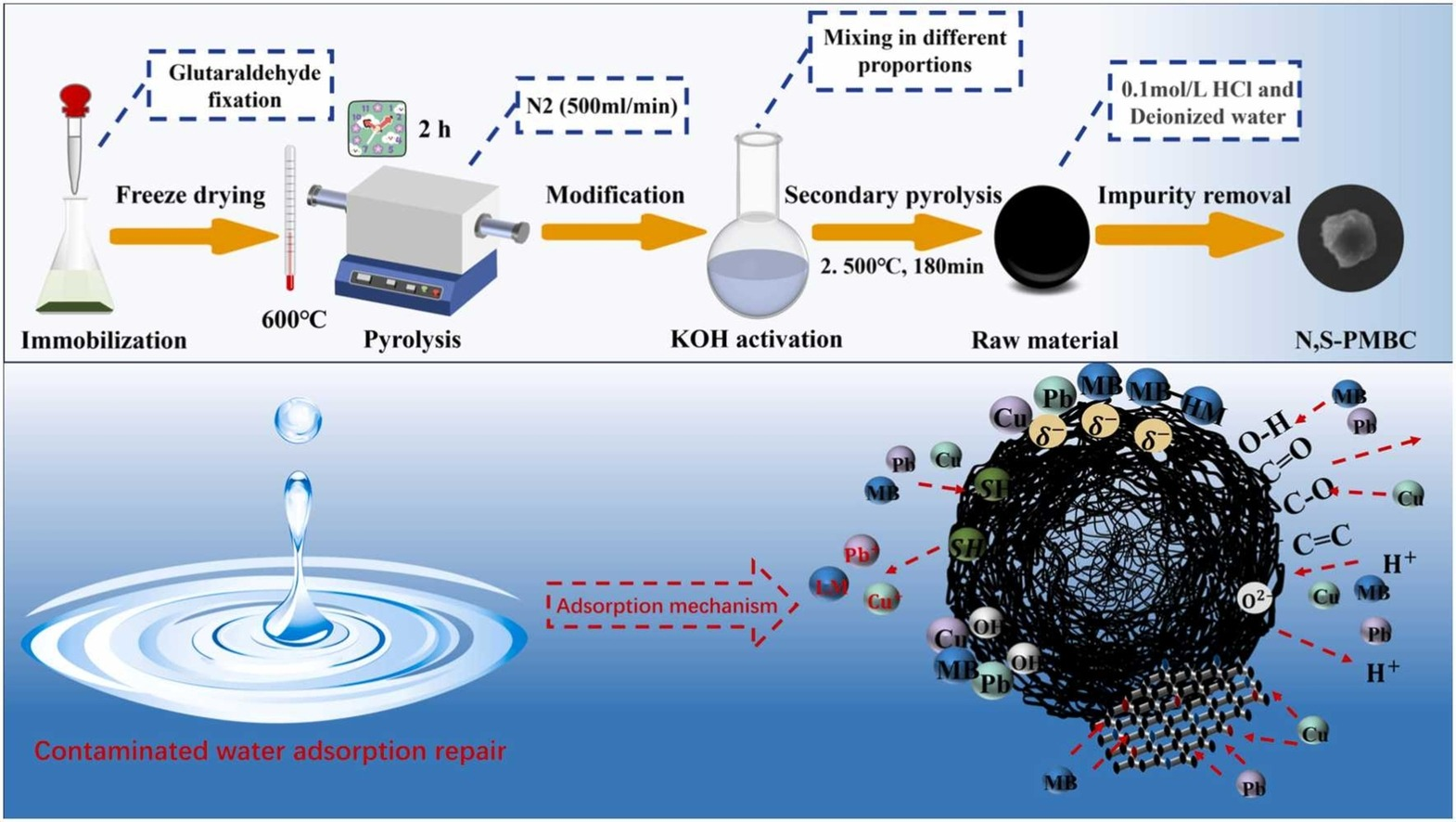
Collaborative governance of various pollutants in water bodies presents a global challenge. This study synthesized nitrogen and sulfur-co-doped micron-biochar (N, S-PMBC) using the pyrolysis properties of endogenous proteins in Chlorella pyrenoidosa. The characterization using SEM, BET, XPS, and other analytical techniques revealed a spherical microstructure with a diameter of 85 μm and a high specific surface area of 2844.42 m²/g. The surface contains many oxygen and nitrogen-rich functional groups (such as Cdouble bondO and pyridine-N) and sulfur-oxygen group (like Sdouble bondO and -SO₃H), exhibiting a pronounced negative charge. N, S-PMBC shows saturation adsorption capacities of 498 mg/g for MB, 192 mg/g for Cu²⁺, and 164 mg/g for Pb²⁺, representing a 100–251 % increase compared to undoped counterparts. Kinetic and thermodynamic analyses showed a spontaneous physicochemical adsorption process (ΔG < −4.45 kJ/mol). Adsorption efficiency under MB-HM coexistence increased exhibited by 143 %, driven by sulfur transformations and synergistic reduction. Thesynergistic adsorption mechanism, based on endogenous sulfur species and the adaptive functions of N, S-PMBC, reduces competitive adsorption while increasing capacity and selectivity, offering a new approach for multicomponent aquatic systems.